ABOUT THE BOOK
The Truth Pill
Since 2004, when the fraud at Ranbaxy, the largest Indian pharmaceutical company at the time first came to light, the Indian pharmaceutical industry and clinical research organizations have been rocked by a series of scandals after investigations by American and European drug regulators. While the West has responded to concerns about quality of “Made in India” medicine by blocking exports from many Indian pharmaceutical companies, the Indian government responded not with regulatory reform but conspiracy theories about “vested interests” working against India.
More worryingly, the Indian state has also turned a blind eye to a far more serious quality crisis in its domestic pharmaceutical market. At times, these quality issues manifest themselves in deaths of Indian citizens as happened in early 2020 when 11 children died in Jammu because of adulterated cough syrup. On other occasions, a dodgy drug approval process has led to the Indian regulator approving sales of drugs that have never been approved by regulators in the developed markets. The result is not just poor health outcomes but outsize profits for pharmaceutical companies manufacturing medicines that have never been validated through scientifically rigorous clinical trials for therapeutic evidence.
These twin crises, in both the domestic and export markets is because India has either outdated regulations or no regulations in some areas. Even the outdated regulations are enforced with kids gloves by drug inspectors and judicial magistrates who are ready to forgive even those whose drugs are found to contain barely any active ingredient or dangerously high levels of bacterial endotoxins. In a race for growth of the pharmaceutical industry, the Indian state has sacrificed scientific rigors and ignored the basic principles of public health. Given India’s position as the pharmacy of the developing world, the failure of the Indian state is a problem for not just India but most of the developing world.
This timely, important and compelling book based on deep research, questions and analyzes the actions of the institutions that are responsible for the safety and efficacy of the Indian drug supply in the context of the historical evolution of the Drugs Act, 1940 from pre-Independence India to the present day. The future of Indian public health lies in responding to the issues raised in this book.
Featured Articles

Big Tech’s contempt for Indian public health
The rise in misleading advertisements by popular platforms is also linked to the immunity that Big Tech enjoys in India
Read More
The medical boundaries for AYUSH practitioners
The core issue is whether they can refer to themselves as doctors and the scope of their medical activities, as this is a subject with consequences for public health in India
Read More
Why states need to act against spurious drugs
Substandard drugs are becoming a political liability, governments must do more to eliminate them from the market
Read More
A bad omen for public trust in vaccines (The Hindu, March 25, 2025)
Multiple vaccine-related petitions that have been filed before courts reflect growing concerns about how vaccines are approved and administered in India
Read More
Getting drunk, on homoeopathy
Alcoholic tinctures marketed in India as homoeopathic remedies are a significant public health hazard
Read More
Turning the clock back on the pharma quality code Read more at: https://www.deccanherald.com/opinion/turning-the-clock-back-on-the-pharma-quality-code-3345535
From a reading of the interim order issued by the court, it appears that these lobbies want to continue manufacturing nutraceuticals in the same facilities where their members are manufacturing drugs.
Read More
Ban this carcinogenic ‘heart-burn’ drug
Last month, the multinational pharmaceutical company, GSK announced a record settlement of $2.2 billion in the United States…
Read More
Regulatory reform stuck in a loop in Health Ministry
The policy initiatives of recall guidelines, good distribution practices and the use of similar brand-names either lack the force of law or are poorly thought through
Read More
Transparency is the right drug for Ayush regulation
A simple regulatory remedy would be for the apex court to mandate state drug controllers to disclose..
Read More
Trials, medical ethics and the orbit of power
In India, the primary guardrails that are supposed to be a check on the abuse of medical ethics do not function
Read More
Govt defends omission of rule restricting Ayurveda drug ads
The Union government on Monday defended in the Supreme Court its August 2023 missive to states and Union territories asking authorities
Read More
The absence of a quality mindset
It took several years of bad publicity — ranging from non-standard quality (NSQ) drugs and counterfeit medicines
Read More
Cartelisation by pharma trade bodies
The penalty imposed by CCI is hardly a deterrent. Also its orders have often been overturned by appellate bodies
Read More
Rx dose of quality control meds
As 2023 ended, the health ministry finally notified a new ‘Good Manufacturing Practices’ (GMP) code, in Schedule M to Drugs and Cosmetics Rules 1945.
Read More
India’s alarming ‘fixed dose combination’ problem
That many of these unapproved or banned FDCs contain antibiotics is cause for concern given the growing antibacterial microbial resistance in India
Read More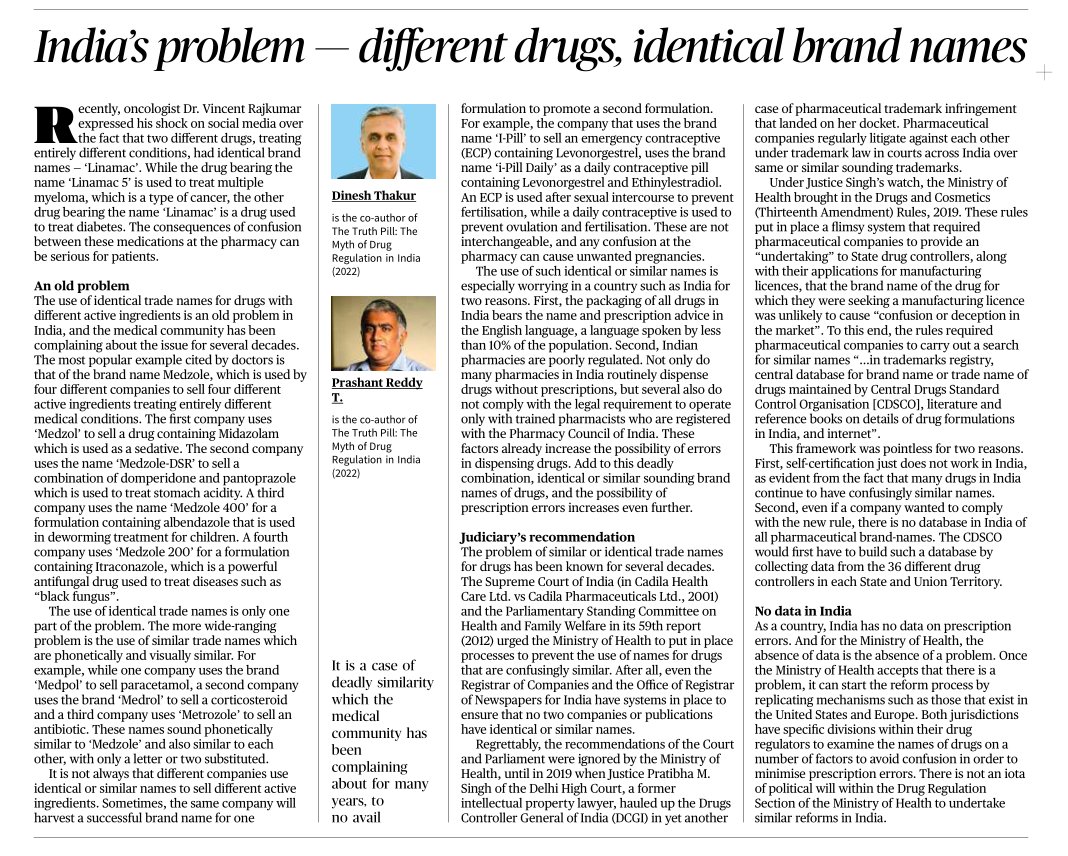
India’s problem — different drugs, identical brand names
It is a case of deadly similarity which the medical community has been complaining about for many years, to no avail..
Read More
India must save the reputation of its pharma industry
It has now been a year since a ‘Made in India’ cough syrup was linked to the deaths of over 70 children in the West African nation..
Read More
The generics-only prescription rule does not make sense when quality of drugs remains suspect first, have a head for meds
The generics-only prescription rule does not make sense when quality of drugs remains suspect.
Read More
फार्मा सेक्टर: निगरानी, निरीक्षण और नियमन के सख्त मानकों की दरकार
जनवरी 2020 में हिमाचल प्रदेश की एक दवा निर्माता कंपनी के कोल्डबेस्ट पीसी कफ सिरप के सेवन के बाद जम्मू में 12 बच्चों की मौत हो गई।
Read More
Make the drug approval files publicly accessible
In the first week of June, the Union health ministry exercised its powers under the Drugs and Cosmetic’s Act…
Read More
Why are Indian drugmakers under the lens?
Since October last year, Indian Pharma companies have been under constant…
Read More
Spare the rod and change the law
The Director General of Health Services (DGHS) issued yet..
Read More
Opinion | India’s Ayush industry needs an entirely new regulatory model
This industry has gotten away with light rules for too…
Read More
State lethargy amidst cough syrup poisoning
A national recall of Coldbest-PC, the medicine behind the…
Read More
When violations aren’t penalised: The legality of the Patanjali advertising tsunami
The ads, published in leading papers like Indian Express…
Read More
Drugs, Medical Devices & Cosmetic Bill: Why the Bill is bad medicine
In its current form, the New Drugs, Medical Devices and Cosmetics Bill…
Read More
The New Drugs Bill: A missed opportunity for reforms in Ayush
The Bill takes regressive steps on the issue of drug safety.
Read More
A new legislation that mirrors the old
The New Drugs, Medical Devices and Cosmetics Bill is…
Read More
Drugs, medical devices & cosmetics bill 2022: A law whose date has expired
India is yet to move away from understanding drug regulation…
Read More
India’s drug approval system is broken but a proposed new law does nothing to fix it
Despite the Drugs Controller General of India having functioned in a…
Read More
New drugs bill is a prescription for disaster
Dinesh Thakur, Prashant Reddy T write: It serves the interests…
Read More